How to get rich combining generic drugs: lessons from Axsome
Also, an experiment in blog post writing
I adapted this blog post directly from a presentation I did for Cure Parkinson’s, a UK charity that’s devoted to, well, Curing Parkinson’s. They’ve been running very cool repurposing trials, and are interested in exploring combination therapies instead of straight repurposing. They asked me to present on how other diseases have approached combination therapies. I thought Axsome Therapeutics would be a particularly interesting company to look at, because they’ve recently been incredibly successful at combining generic drugs for novel indications.
Instead of doing this as a proper blog post, I thought I’d just copy-paste my slides into Substack, because I think they largely speak for themselves. Let me know if this works.
Also, this post is too long for email, so click on the title to be taken to the web link to read it in full.
Since 2020, 5 of the ~250 drugs approved by the FDA have been combinations of generic drugs.
2 of those have been by Axsome Therapeutics, and have been great commercial successes.
But how? Let’s figure out the questions that need to be asked and Axsome’s answers.
1. Figure out your mechanisms: what does each drug contribute individually?
2. Rationalize your combination: why do they need to be combined?
3. Make your formulations unique: why wouldn’t doctors just prescribe each drug separately?
4. Build your patent fortress: how do you prove this combination is novel and non-obvious?
5. Plan your trials: how do you take advantage of the data in the literature?
6. Work with payers and regulators early: how do you convince stakeholders to back the combination?
Let’s look specifically into how Axsome did it with Auvelity (dextromethorphan-bupropion).
1. Figure out your mechanisms: what does each drug contribute individually? Dextromethorphan is a rapid antidepressant and treatment for agitation in dementia. Bupropion is a long acting antidepressant and treatment for smoking cessation.
2. Rationalize your combination: why do they need to be combined? PD and PK synergy.
3. Make your formulations unique: why wouldn’t doctors just prescribe each drug separately? 45 mg DXM and 105 mg ER bupropion are unique and impossible to obtain generically.
Patent fortress: how do you prove this combination is novel and non-obvious to get your patents and intellectual protection? You can get patents on method-of-use, formulation, and dosage form.
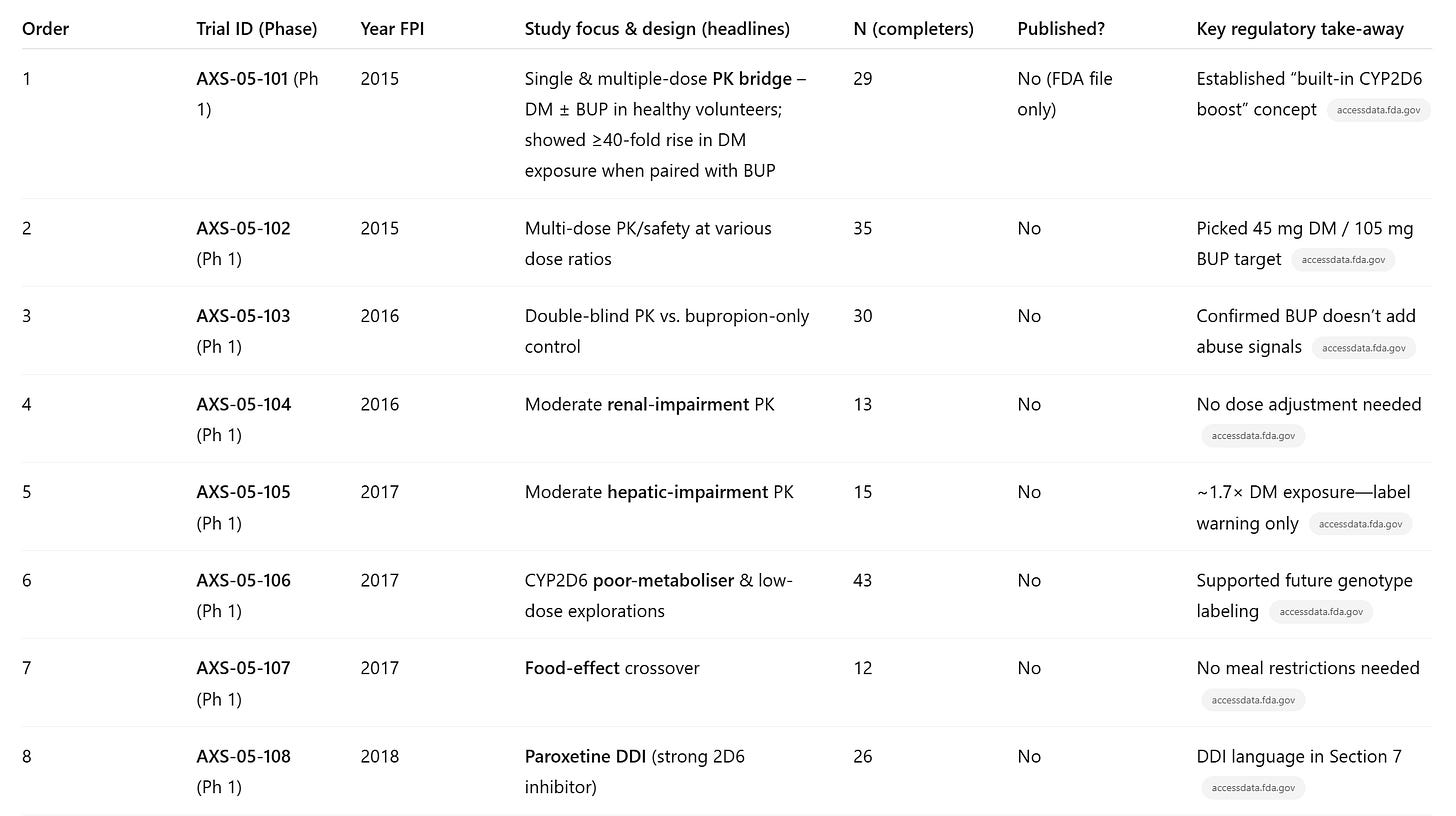
Plan your trials: how do you take advantage of the data in the literature to build out a comprehensive trial plan and get quickly to market? Do many phase 1 trials of the combination in healthy volunteers. They are the most important, because you know your mechanism of action and are confident on safety, you just don’t know how the drugs interact.
Have patience! The stock didn’t take off until Axsome got good phase 2 results, which meant they had 4 years of Phase 1 trials that didn’t budge the stock price.
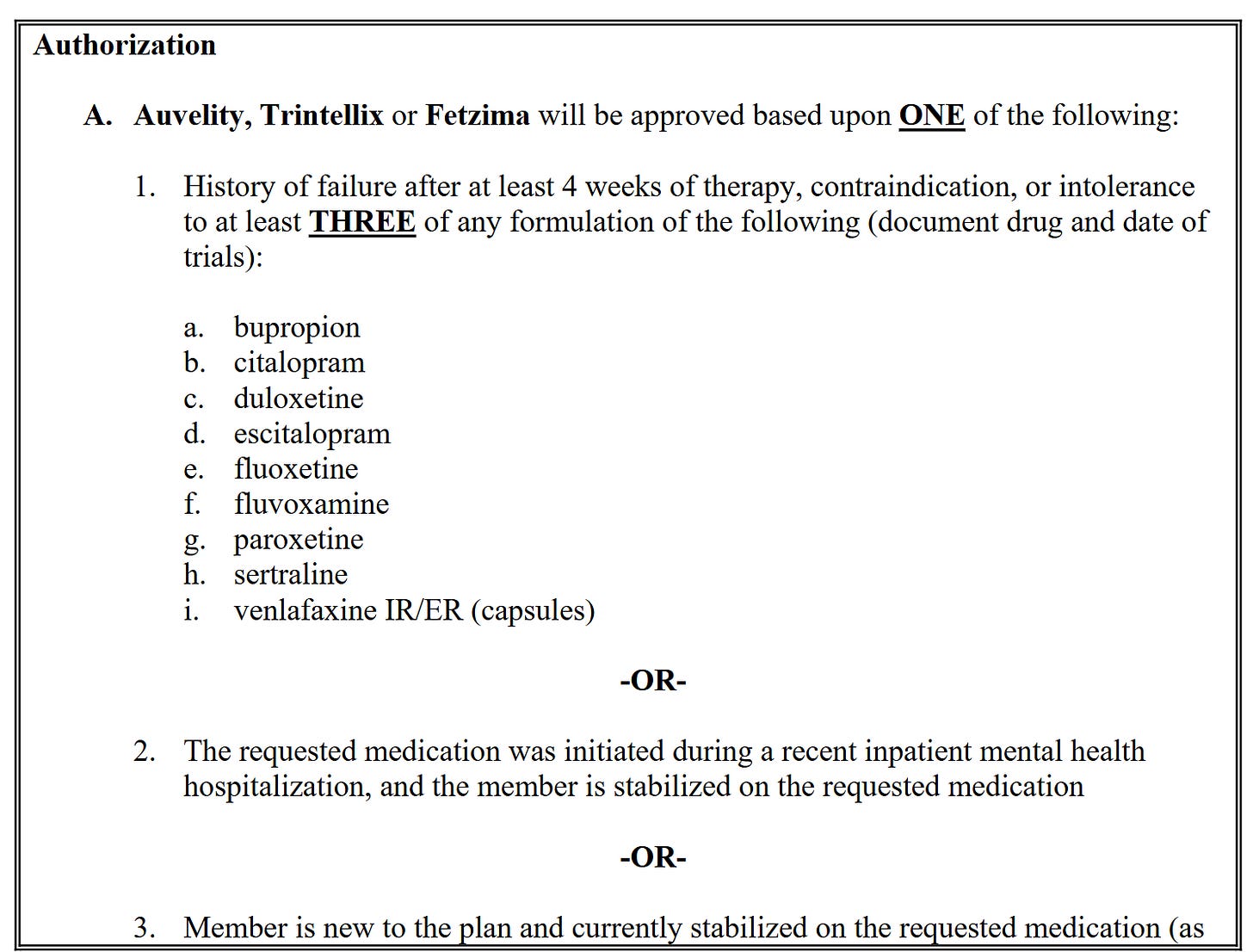
Working with payers and regulators early: how do you convince stakeholders to back the combination? The FDA needs to know why you need exclusivity and how you plan to handle it, and payers need to know where your combo fits in with established treatment plans (i.e. why they should pay for it).
Working with regulators
Working with payers
Note: all of these questions should be answered to the greatest extent possible before doing development. One of the biggest advantages of generic drugs is that you have a lot of information from the literature before you do any work yourself. You should take advantage of it!














This was revelatory; great post!