Bacterial vaginosis: we contain multitudes
Multitudes occasionally centered around our genitals.
As a service to the impatient reader, the tl;dr is that you can cure recurrent bacterial vaginosis by antibiotics combined with intravaginal vitamin C and/or intravaginal probiotics. Limiting the amount of bleeding you undergo every month (i.e. by using hormonal birth control) also helps. But seriously, read the rest of the post, because bacterial vaginosis is very interesting.
In 10th grade, an English teacher caught some friends of mine doodling penises on a school table. In the midst of yelling at them for defacing school property, he asked, “You know, what is it about penises that is so fascinating to you guys? Why do you never draw vaginas? Are they just not as interesting?”
That question has haunted me for the decade plus since that event1. What I’ve concluded is that vaginas are not not as interesting as penises. I think, on the contrary, they’re too interesting. They’re too mysterious. Now that I’m older and hopefully more mature, I think I’m finally ready to tackle some of the mysteries of the vagina. Specifically, I want to discuss bacterial vaginosis.

Now, I know what you’re thinking. Bacterial vaginosis (BV) is generally not the sort of thing that one wants to discuss. Not only is it generally regarded as gross and embarrassing by the sufferers, it’s also not regarded as that serious an illness by the world at large. Nobody’s ever told a friend, “I’m so proud of you for successfully fighting your BV.”
So why do I want to discuss it? Well, first of all, because it’s this interesting mix of very common but poorly understood. Somewhere north of 30% of women probably have it at any given time2, but we still don’t really know what causes it.
Second, because BV’s not your average disease. BV is a perturbation of the microbiome of the vagina, which means that the “normal” bacteria of the vagina have been replaced by some kind of abnormal bacteria. This is very different from your average infection, which is a much more clear cut case of “foreign cells enter into your body and start wrecking stuff”. Instead, discussing BV means discussing really interesting questions of what’s normal, what’s abnormal, and how and why one flips between the two. It forces us to acknowledge that our body is not one but many, and try to figure out what that means for health.
Let’s start with the obvious question, first. What is BV?
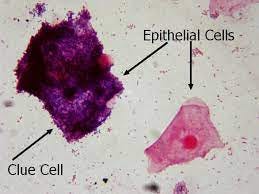
Well, there’s two ways of defining it. There’s the mostly symptomatic Amsel criteria, which requires 3 of the 4 criteria to be present: a thin white or gray discharge, a change in pH to more alkaline, a fishy smell, and/or bacteria on the vaginal epithelium, usually (and maybe always) Gardnerella vaginalis. Alternatively, there’s the etiological (i.e. causal) Nugent criteria, which measures a shift from the “normal” Lactobacilli in the vagina to other sorts of bacteria.
Which criteria you use says a lot about what you consider BV to be already. If you just think of BV as “something gross is happening to my/my patient’s vagina and I’d like it to stop”, then you’d use the Amsel criteria. On the other hand, if you think of BV as a shift away from a “normal” microbiome to an “abnormal” microbiome, you’d use the Nugent criteria. In the latter view, it doesn’t matter if there are any symptoms that the patient might be bothered by. It’s still a disease even if the patient doesn’t care.
Most studies end up using a mix of both and don’t think about it further. However, as you might expect, the sort of benevolent paternalism inherent in using something like the Nugent criteria and its assumption of what makes a “normal” vagina has led to a minority view that BV might not be a disease at all. Perhaps, this minority view suggests, BV is just a different “normal” state that vaginas can be in, in the same way both straight and curly hair are just different “normal” states that hair can be in.
If that analogy seems uncomfortably race-adjacent to you, that’s no accident. One of the mysteries about BV is that its prevalence varies a lot by race and location. Black women in the US are two times as likely to have BV as white women (50% vs 23.2%), while Mexican American women are about 1.5 times as likely (32%). Women in Tibet apparently have BV levels of 60%, while white women in Barcelona have BV levels of 6.4%.
So, this minority view suggests, we are really just stigmatizing poor, non-white women for no good reason. Maybe we should just accept that some vaginas smell fishy or have discharge, and stop trying to fix them.
I’m sympathetic to this view, but only up to a point. This minority view is correct that the change in the vaginal microbiome in and of itself isn’t harmful, but, as the Amsel criteria suggests, a fishy smell and discharge are not the only consequences of BV. There’s also a change in pH, from a relatively acidic 4.0-4.5 to a more basic 5.0-5.5.
This relatively small change is very important, because changing the pH from 4.0 to 5.0 makes the vagina a much more hospitable environment for a range of pathogens. So, for example, there’s strong evidence that BV makes it more likely for a woman to catch chlamydia or gonorrhea if she has sex with an infected partner, and, vice versa, that both chlamydia and gonorrhea prefer more alkaline vaginal environments3.
Anything that’s good for transmitting chlamydia is probably bad overall, in my estimation. So, I think we can safely say that BV is bad.
Now that that hot take is out of the way, we next have to address another difficult question. How does one get BV?
Well, if we go back to the etiological part of the Amsel and Nugent criteria, it’s most natural to think of it as some kind of infection, specifically an infection of Gardnerella vaginalis (GV), which results in those distinctive “clue cells” on the epithelium. And there are definitely parts to that which make sense.
First, there’s the fact that BV can be cured, at least temporarily, by antibiotics. Second, back in the 50s and 60s, when they did challenge trials in BV, they did have limited success in giving women BV by infecting them with GV4. Third, it does seem possible to sexually transmit BV in the wild. Women who have sex with women are more likely to have BV than women who have sex with men, and, if you’re a lesbian with a sex partner with BV, you’re more likely to contract it than if you’re a woman whose sex partner does not have BV.
But all of these come with caveats. For antibiotics, more than 50% of women experience a remission in 6-12 months. In those challenge trials, it was very difficult to infect women with GV alone. Infecting women with vaginal fluid from a woman with BV was much more successful. And for sexual transmission, men who have GV in their urethra don’t seem to be able to transmit it to women very easily.
We also get the issue that GV on its own doesn’t seem that bad. Almost all women have some amount of GV, and even some women with high levels of GV don’t necessarily have BV5. In fact, GV on its own cannot produce a fishy smell.
So, we can’t really model GV as an STI in the same way as we can something like chlamydia. Instead, we can probably think of GV instead as an endemic bacteria that can be picked up from a lot of different places, and can live in a lot of different places, including the pharynx, rectum, and urinary tract. Unlike Lactobacillus, it prefers iron-rich blood and slightly higher pHs. However, much like Lactobacillus, it promotes the pH that it prefers.
Most of the time, Lactobacillus is able to be dominant in the vagina by producing antimicrobial substances and promoting low pH by producing lactic acid. However, sometimes, the situation changes to favor GV dominance. This might be because of a sudden insult to the Lactobacillus population, like a change in vaginal microenvironment or a new antibiotic. Or, it might be a sudden boost to the GV population, like the introduction of a large amount of GV or a lot of iron-rich blood. If the former, the GV can start producing a biofilm and raising the pH, further promoting its growth and limiting that of Lactobacillus.
Antibiotics might then appear to be effective if they successfully reduce the population of GV. However, this only works if the GV itself is eliminated (rather than, say, the odor causing bacteria), and if the vaginal microenvironment is then made hospitable to Lactobacillus again, possibly by Lactobacillus itself.
One very obvious way to make the vaginal microenvironment more hospitable to Lactobacillus than to GV is to limit the amount of blood available. GV prefers blood to grow; Lactobacillus does not. Hormonal birth control, which can limit or stop periods, is therefore associated with a significantly reduced risk of BV. This may also explain the difference in prevalence of BV between white and black women, as white women are more likely to be on hormonal contraception.
The other thing that you might think of in terms of restoring the vaginal microenvironment is making it more acidic again. The most common way of doing this is douching, or washing the vagina out. Unfortunately, this isn’t the best way of doing it. First of all, most women who douche will douche regardless of whether they have BV or not, which unnecessarily changes the microenvironment. Second, many douches aren’t particularly acidic or have other unnecessary components to smell good, which defeats the purpose. These factors combined mean that regular douching is actually associated with BV (although, interestingly, douching before or after sex is associated with a significant reduction in chlamydia or gonorrhea).
But, other acidic treatments do work well. Intravaginal boric acid has some good anecdotal support, and intravaginal vitamin C tablets actually worked very well in a double-blind trial (55% of women cured with treatment vs 25% cured with placebo). Intravaginal vitamin C tablets also worked great to prevent recurrence of bacterial vaginosis when women inserted them for 6 days after menses for 6 monthly cycles (16.2% recurrence for treatment in the 6 months vs. 32.4% recurrence for placebo).
And then, of course, you can try to give a headstart to Lactobacillus with intravaginal probiotics, which also worked well in a double-blind trial (16% recurrence for treatment vs. 45% for placebo) as well as in a Cochrane review. As with all probiotic trials, though, the authors warn of the prevalence of bad quality probiotics, which either fail to make sure their bacteria are alive or fail to make sure that the probiotic contains the bacteria at all.
All of this is useful enough for practical purposes. However, what I think would be especially interesting is taking this knowledge and using it for the other super important microenvironment in the body that’s much harder to access: the digestive tract. There have been a ton of studies and papers done on probiotics, antibiotics, and microbiota transplants for the digestive tract, but there still aren’t firm conclusions on what to do. And, more importantly, many digestive tract issues remain, uh, intractable.
Are there digestive tract equivalents of vitamin C tablets and boric acid? Should we be inserting probiotics rectally instead of swallowing them? Is a fecal transplant equivalent to a vaginal fluid transplant? Can we (and should we) nurture the Lactobacillus in the stomach by using lessons from the vagina?
There are only so many places where our commensal bacteria are important for our health, and few as easily accessible as the vagina. This could at last be our chance to explore not only the mysteries of the vagina, but ourselves.
Not really.
Wikipedia gives a rate of “5 to 70%”, which is an entirely useless range but reflects the fact that it is definitely common, but that the prevalence varies so much from region to region it’s hard to get a good grasp on how common it is overall.
There’s also weaker evidence that women with BV are more likely to have HPV, UTIs, and HIV. However, it’s really hard to piece out correlation and causation with these ones. Does HPV cause BV, BV cause HPV, or something else (e.g. poor hygiene) cause both?
However, in the chlamydia/gonorrhea studies, they’re examining women at STD clinics who have had sex with an infected partner. So, everyone at the clinic is already at risk for an STD, and it’s only the immediate sexual environment that makes a difference.
Weirdly, reporting regular condom use did not seem to make much of a difference, as 10% of condom users and 10% of non-condom users both got gonorrhea, and 7% of condom users and 11% of non-condom users got chlamydia. This was a notably poor population with only 23% of people on non-Medicaid insurance, so people might just be lying about condoms or using them wrong.
The 50s and 60s were a different, worse time in medical ethics. Granted, they probably thought BV was harmless except for the bad smell, but you think you’d take precaution before just jumping in and infecting people. Also, they did some of these studies on pregnant women, with absolutely no idea what this would do to the fetus.
Although there might still be a change to the pH, which would be bad, as aforementioned.